Advertisements
Advertisements
Question
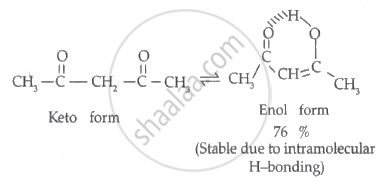
Assertion A: Enol form of acetone [CH3COCH3] exists in < 0.1% quantity. However, the enol form the acetyl acetone [CH3COCH2OCCH3] exists in approximately 15% quantity.
Reason R: Enol form of acetyl acetone is stabilized by intramolecular hydrogen bonding, which is not possible in enol form of acetone.
Choose the correct answer:
Options
A is true but R is false.
Both A and R are true but R is the correct explanation of A.
A is false but R is true.
Both A and R are true but R is not the correct explanation of A.
Solution
Both A and R are true but R is the correct explanation of A.
Explanation:
\[\begin{array}{cc}
\phantom{...}\ce{O}\phantom{................}\ce{OH}\phantom{}\\
\phantom{}||\phantom{.................}|\phantom{}\\
\ce{\underset{Keto form}{CH3-C-CH3}<=>\underset{\underset{<0.1{\%}}{Enol form}}{CH2=C-CH3}}\\
\end{array}\]