Advertisements
Advertisements
Question
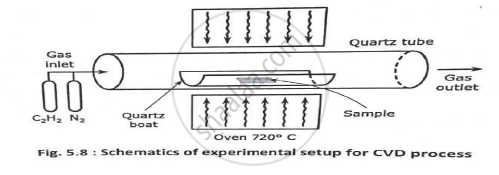
Explain CVD method for production of CNTs.
Solution
This is very good method from large scale production of carbon nanofiber SWNT, MWNT. Hydrocarbons (e.g., methane, ethane) are allowed to decompose over metal catalyst (e.g., Co, Fe) to produce CNT. Typical yield for CVDare approximately 30% _ This process includes production of large amount of CNT's by CVD of acetylene over cobalt and iron. _ Ethylene can be used with reaction temperatures of 54S°C for nickel catalyst CVO and 900°C for an uncatalyzed process that produces carbon nanostructure with open ends. Methane can also be used as carbon source for synthesization _ catalytic decomposition of H2/CH4 mixture over cobalt, nickel, and iron is used to obtain yields of SWNTs at 1000°C The usage ofH/CH4 atmosphere between a non-reducible oxide such as Al2O3 or MgAl2O4 and one or more transition metal oxides can produce the composite powders containing well dispersed CNTs. Thus, higher proportion of SWNTs and lower proportion ofMWNTs can be achieved using the decomposition of CH4 over the nanoparticles. Thermal catalytic decomposition of hydrocarbon has become an active area of research and can be a promising route for the bulk production of CNTs. The removal of the catalyst support via an acid treatment which sometimes could destroy the original structure of the carbon nanotube is an issue in this synthesis route. However, alternative catalyst supports that are soluble in water have proven effective for nanotube growth.