Advertisements
Advertisements
Question
Explain with an example of Antiseptics.
Solution
- Antiseptics are used to sterilise surfaces of living tissue when the risk of infection is very high, such as during surgery or on wounds.
- Commonly used antiseptics include inorganics like iodine and boric acid or organics like iodoform and some phenolic compounds.
e.g.
- Tincture of iodine (2-3% solution of iodine in an alcohol-water mixture) and iodoform serve as powerful antiseptics and is used to apply on wounds.
- A dilute aqueous solution of boric acid is a weak antiseptic used for eyes.
- Various phenols are used as antiseptics. A dilute aqueous solution of phenol (carbolic acid) is used as antiseptic; however, phenol is found to be corrosive in nature. Many chloro derivatives of phenols are more potent antiseptics than the phenol itself. They can be used with much lower concentrations, which reduce their corrosive effects.
- Two of the most common phenol derivatives in use are trichlorophenol (TCP) and chloroxylenol (which is an active ingredient of antiseptic dettol).
- Thymol obtained from oil of thyme (a spice plant) has excellent non-toxic antiseptic properties.
APPEARS IN
RELATED QUESTIONS
Identify the functional group in the following molecule:
Aspirin
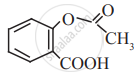
Identify the functional group in the following molecule:
Paracetamol
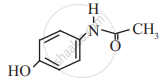
Give two differences between the Disinfectant and antiseptic.
Match the pairs.
| A group | B group |
| A. Paracetamol | a. Antibiotic |
| B. Chloramphenicol | b. Synthetic detergent |
| C. BHT | c. Soap |
| D. Sodium stearate | d. Antioxidant |
| e. Analgesic |
Name two drugs which reduce body pain.
Explain with an example of Anionic detergents.
Explain with an example of Nonionic detergents.
Identify the functional group in the following molecule:
Penicillin

Identify the functional group in the following molecule:
Chloramphenicol
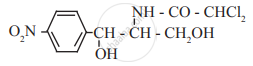
Answer in one sentence.
Name the pain killer obtained from acetylation of salicylic acid.
Answer in one sentence.
Draw the structure of chloroxylenol.
Answer in one sentence.
Draw the structure of salvarsan.
Answer in one sentence.
Write molecular formula of Butylated hydroxytoluene.
Write the molecular formula and name of:

Write two examples of the following:
- Analgesics
- Antiseptics
- Antibiotics
- Disinfectant
Butylated hydroxy anisole is a/an ______.
Commonly used antiseptic ‘Dettol’ is a mixture of ______.
Which of the following processes is not used to preserve the food?
What is the combining ratio of glycerol and fatty acid when they combine to form triglyceride?
Which of the following is a hypnotic drug?
Which of the following is a bactericidal antibiotic?
Which of the following is not a tranquilizer?
