Advertisements
Advertisements
Question
Hybridisation of \[\ce{[Ni(CN)4]^{2-}}\] is ______.
Options
dsp2
sp3d2
d2sp3
sp3
MCQ
Fill in the Blanks
Solution
Hybridisation of \[\ce{[Ni(CN)4]^{2-}}\] is dsp2.
Explanation:
Ni = 1s2, 2s2, 2p6, 3s2 3p6 3d8 4s2
Ni2+ = 1s2, 2s2, 2p6, 3s2 3p6 3d8, 4s0 4p0
Ni2+ : 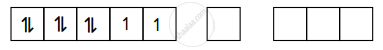
\[\ce{[Ni(CN)4]^{2-}}\] :
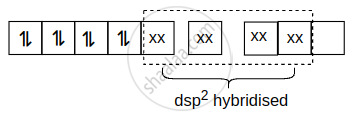
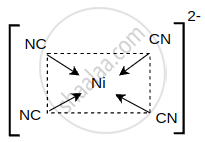
shaalaa.com
Hybridization
Is there an error in this question or solution?
