Advertisements
Advertisements
Question
The variation of vapour pressure of different liquids with temperature is shown in figure.
(i) Calculate graphically boiling points of liquids A and B.
(ii) If we take liquid C in a closed vessel and heat it continuously. At what temperature will it boil?
(iii) At high altitude, atmospheric pressure is low (say 60 mm Hg). At what temperature liquid D boils?
(iv) Pressure cooker is used for cooking food at hill station. Explain in terms of vapour pressure why is it so?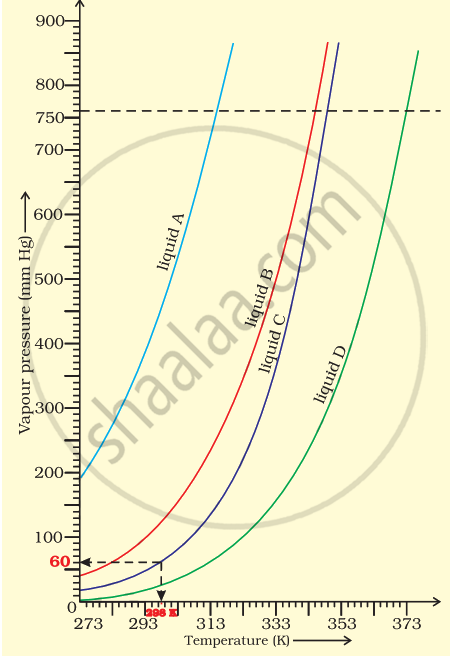
Solution
(i) Boiling point of liquid A ≃ 315 K, B ≃ 345 K
(ii) In a closed vessel, liquid C will not boil because pressure inside keeps on increasing.
(iii) Temperature corresponding to 60 mm ≃ 313 K.
(iv) A liquid boils when its vapour pressure becomes equal to atmospheric pressure. At hill station, atmospheric pressure is low. Therefore, liquid boils at a lower temperature and cooking is not perfect. In a pressure cooker the pressure inside increases and the liquid boils at a higher temperature.
APPEARS IN
RELATED QUESTIONS
Atmospheric pressures recorded in different cities are as follows:
| Cities | Shimla | Bangalore | Delhi | Mumbai |
| p in N/m2 | 1.01 × 105 | 1.2 × 105 | 1.02 × 105 | 1.21 × 105 |
Consider the above data and mark the place at which liquid will boil first.
Which of the following changes decrease the vapour pressure of water kept in a sealed vessel?
(i) Decreasing the quantity of water
(ii) Adding salt to water
(iii) Decreasing the volume of the vessel to one-half
(iv) Decreasing the temperature of water
Pressure exerted by saturated water vapour is called aqueous tension. What correction term will you apply to the total pressure to obtain pressure of dry gas?
Which of the following has the dimension of ML0T–2?
