Advertisements
Advertisements
Question
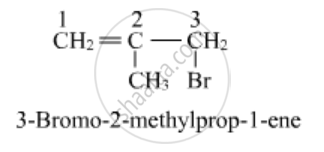
Write the structure of 3-Bromo-2-methylprop-1-ene
Solution
APPEARS IN
RELATED QUESTIONS
Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Williamson's synthesis of preparing dimethyl ether is a/an ____________.
Which of the following cannot be made by using Williamson Synthesis:
The major product [B] in the following reactions is:
\[\begin{array}{cc}\ce{CH3}\phantom{..................................}\\|\phantom{.....................................}\\\ce{CH3 - CH2 - CH - CH2 - OCH2 - CH3 ->[HI][Heat] [A] alcohol ->[H2SO4][\Delta] [B]}\end{array}\]
Write the name of reagent and equation for the preparation of the following ethers by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of the reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Identify the product (s) is/are formed when 1-methoxy propane is heated with excess HI. Name the mechanism involved in the reaction.
Write the name of the reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the names of reagents and equations for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
