Advertisements
Advertisements
प्रश्न
Account for the following:
pKb of aniline is more than that of methylamine.
उत्तर १
pKb of aniline is more than that of methylamine:
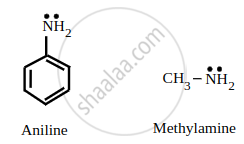
Aniline undergoes resonance, and as a result, the electrons on the \[\ce{N}\]-atom are delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate.
On the other hand, in the case of methylamine (due to the +I effect of the methyl group), the electron density on the \[\ce{N}\]-atom is increased. As a result, aniline is less basic than methylamine. Thus, pKb of aniline is more than that of methylamine.
उत्तर २
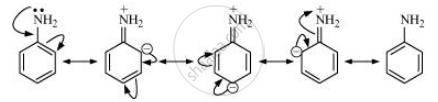
In aniline, the lone pair of electrons on the \[\ce{N}\]-atom is delocalised over the benzene ring.
As a result, electron density on the nitrogen atom decreases. Whereas in \[\ce{CH3NH2 + I}\] effect of \[\ce{- CH3}\] group increases the electron density on the \[\ce{N}\]-atom. Therefore, aniline is a weaker base than methylamine, and hence its pKb value is higher than that of methylamine.
APPEARS IN
संबंधित प्रश्न
Which among the following molecular formulae represents urotropine?
(a) C6H12N4
(b) C6H24H4
(c) C6H12N4O2
(d) C6H24N4O2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)2CHNH2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)3CNH2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
C6H5NHCH3
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3CH2)2NCH3
Give one chemical test to distinguish between the following pair of compounds.
Aniline and benzylamine
Account for the following:
Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
How will you convert Ethanoic acid into methanamine
How will you convert Methanol to ethanoic acid
How will you convert Methanamine into ethanamine
How will you convert Propanoic acid into ethanoic acid
Accomplish the following conversions - Aniline to 2,4,6-tribromofluorobenzene
Accomplish the following conversions - Aniline to benzyl alcohol.
Complete the following reactions:
`C_6H_5NH_2 +H_2SO_4(conc.) ->`
Complete the following reactions:
`C_6H_5NH_2 + (CH_3CO)_2O->`
Do the following conversions in not more than two steps :
Propanone to Propene
Write the structure of 2,4-dinitrochlorobenzene
Identify the incorrect IUPAC name.
An organic compound (A) with molecular formula C3H7NO on heating with Br2 and KOH forms a compound (B). Compound (B) on heating with CHCl3 and alcoholic KOH produces a foul-smelling compound (C) and on reacting with C6H5SO2Cl forms a compound (D) which is soluble in alkali. Write the structure of (A), (B), (C) and (D).
