Advertisements
Advertisements
प्रश्न
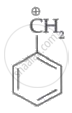 |
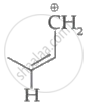 |
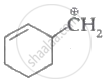 |
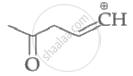 |
| (I) | (II) | (III) | (IV) |
Among the given species the resonance stabilised carbocations are:
विकल्प
(III) and (IV) only
(I), (II) and (III) only
(I), (II) and (IV) only
(I) and (II) only
उत्तर
(I) and (II) only
Explanation:
The delocalization of electrons within a molecule is depicted by resonance structures. While constructing a resonant structure, the molecule's structure, molecular formula, and overall number of electrons all remain the same. The connections, which might take the shape of single, double, or triple bonds, are the only aspect of resonance structure that differs.
Only structure (I) and (II) shows resonance as they fulfil all requirement for it.
The resonance structure of species (I) can be represented as follows:

The resonating structures of species (II) is shown below.

Therefore, the resonance stabilized carbocations are species (I) and (II).
