Advertisements
Advertisements
प्रश्न
Balance the following equation. Also name the product formed.
CO2 + C → CO
उत्तर
CO2 + C → 2CO Corbon monoxide
APPEARS IN
संबंधित प्रश्न
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are non-metals.
Balance the following chemical equation:
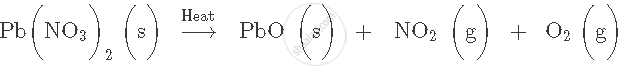
Write word equation for the following skeletal equation:
\[\ce{Na + O2 -> Na2O}\]
Balance the following equation:
Zn + KOH → K2ZnO2 + H2
Write the balanced chemical equation of the following reaction.
silver nitrate → silver + nitrogen dioxide + oxygen
Write the balanced chemical equation of the following reaction.
potassium dichromate + hydrochloric acid → Potassium chloride + chromium chloride + water + chlorine
When a potassium iodide solution is added to a solution of lead (II) nitrate in a test tube, a precipitate is formed.
What is the colour of this precipitate? Name the compound precipitated.
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – lead [IV] oxide.
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
6NaOH + 3Cl2 → 6NaCl + NaClO3 + 3H2O
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
PbO2 +4HCl → PbCl2 + H2O + Cl2
