Advertisements
Advertisements
प्रश्न
Can we use conc. HCl in place of Conc. H2SO4 ?
उत्तर
No, Concentrated HCl cannot be used in the place of concentrated H2SO4 because HCl is more volatile than HNO3 and hence nitric acid vapour will carry HCl vapours.
APPEARS IN
संबंधित प्रश्न
Write balanced equations for Action of heat on a mixture of copper and concentrated nitric acid
Name the Following:
Gas obtained by treating manganese with 1% nirtric acid.
What is meant by the following term ?
Decrepitation
What is the purpose of Conc. H2SO4 in the above preparation?
Choose the correct answer from the option given below :
Nitric acid on standing develops brownish colour which may be attributed to the presence of :
Give reason for the following:
Commercial concentrated nitric acid is yellow in colour, but when it is dilute with water, it turns colourless.
Give one chemical test for nitric acid.
Write balanced equation for the reaction between nitrogen and oxygen, when lightning strikes.
State, what is observe when nitric acid is kept in a reagent bottle for a long time.
Copy and complete the following table relating to important industrial processes output refers to the product of the process not the intermediate steps.
| Name of process | Inputs | Catalyst | Equation for catalysed reaction | Output |
| Haber process | Hydrogen + | |||
|
Ammonia + |
Nitric acid |
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
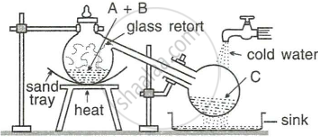
- Name A (a liquid), B (a solid), and C (a liquid). (Do not give the formulae).
- Write an equation to show how nitric acid undergoes decomposition.
- Write the equation for the reaction in which copper is oxidised by concentrated nitric acid.
