Advertisements
Advertisements
प्रश्न
Convert the following reaction into a balanced chemical equation:
Ammonia to nitric oxide using oxygen and platinum catalyst.
उत्तर
\[\ce{4NH3 + 5O2 ->[800^\circ C][Pt] 4NO + 6H2O + Δ}\]
APPEARS IN
संबंधित प्रश्न
Give a balanced chemical equation for Action of conc. Nitric acid on Sulphur
State one relevant observation for the following:
When crystals of copper nitrate are heated in a test tube.
What is aqua fortis?
Identify the substance underlined:
The dilute acid which is an oxidizing agent.
Write down the word equation or balanced equation for the action of concentrated nitric acid on copper.
Choose the correct word from the given options to complete the sentence :
Sulphur can be converted to sulphuric acid using __________ nitric acid.
Write equation to show the reaction between the following:
Copper oxide and dilute nitric acid.
Write a balanced equation for following :
Action of cold and dilute nitric acid on copper
From the formulae listed below, Choose, one, corresponding to the salt having the given description:
AgCl, CuCO3, CuSO4.5H2O, KNO3, NaCl, NaHSO4, Pb(NO3)2, ZnCO3, ZnSO4, 7H2O.
On heating this, salt changes from green to black.
The diagram given below is a representation of the Industrial preparation of Nitric acid by Ostwald’s process. With respect to the process answer the following questions:
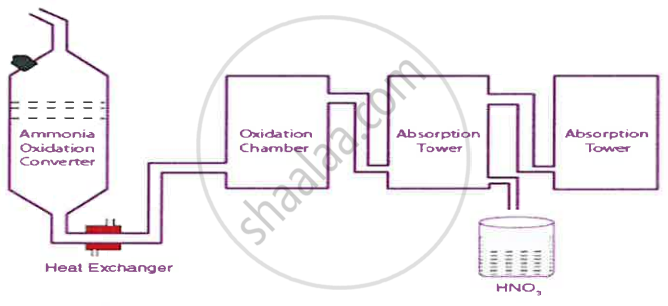
- Write the temperature and the catalyst required during the catalytic oxidation of ammonia.
- Give balanced chemical equation for the reaction occurring duringthe conversion of nitrogen dioxide to nitric acid.
