Advertisements
Advertisements
प्रश्न
Define calorimetry ?
उत्तर
The measurement of the quantity of heat is called calorimetry.
APPEARS IN
संबंधित प्रश्न
What is the principle of Calorimetry?
What is a calorimeter?
1.0 kg of water is contained in a 1.25 kW kettle. Calculate the time taken for the temperature of water to rise from 25° C to its boiling point of 100°C. Specific heat capacity of water = 4.2 J g-1K-1.
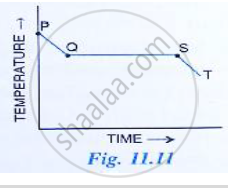
Fig 11. 11 shows the variation in temperature with time when some wax cools from the liquid phase to the solid phase.
(i) In which part of the curve, the wax is in liquid phase?
(ii) What does the part QS of the curve represent?
(iii) In which part of the curve, the wax will be the in the liquid as well as solid phase?
(iv) In which part of the curve, the wax is in solid phase?
How much steam at 100oC must be passed into 120 g of water at 20oC to raise the temperature to 40oC?
40 g of ice at -16°C is dropped into water at 0°C, when 4 g of water freezes into ice. If specific heat capacity of ice is 2100 J/kg°C, what will be the latent heat of fusion of ice?
Name two factors on which the heat energy librated by a body on cooling depends.
20 g of ice at 0°C is added to 200g of water at 20°C. Calculate the drop in temperature ignoring the heat capacity of the container. (Specific latent heat of ice = 80 cal/g)
