Advertisements
Advertisements
प्रश्न
Discuss the variation of conductivity and molar conductivity with concentration.
उत्तर
Both conductivity and molar conductivity change with the change in concentration of electrolyte. Conductivity always decreases with decreasing the concentration of both weak and strong electrolytes. This can be explained by the fact that with dilution, the number of ions carrying electric current per unit volume decreases. The conductivity of a solution at any concentration is the conductivity of a unit volume of the solution placed between two platinum electrodes having a unit cross-sectional area and situated at unit distance from each other.
This is clear from the following equation:
C = `"κA"/"l"` = κ ...(Both A and I are in appropriate units m or cm)
The molar conductivity of a solution at a given concentration is the conductivity of volume V of the solution containing one mole of electrolyte dissolved in it and placed between two electrodes of cross-sectional area A, located at a unit distance from each other. So,
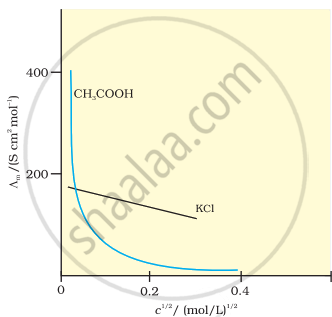
Plot of c1/2 versus molar conductivity for potassium chloride (a strong electrolyte) in aqueous solution.
∧m = `"κA"/"l"` = κ
Since l = 1 and A = V (volume in which one mole of electrolyte is dissolved.)
∧m = κ V
Molar conductivity increases with decreasing concentration. This is because the total volume (V) in which one mole of electrolyte is present also increases. It has been found that on dilution of the solution, the increase in volume is much more than the decrease in κ.
Strong Electrolytes: For strong electrolytes, the value of Δm increases gradually with dilution and it can be represented by the following equation:
∧m = `∧_"m"^0 - "Ac"^(1//2)`
It can be seen that if ∧m is plotted against c1/2, we get a straight line with intercept A and slope equal to ‘A’. The value of constant ‘A’ at a given solvent and temperature depends on the type of electrolyte, i.e., on the charges of the cation and anion produced on dissociation of the electrolyte in solution. Thus, \[\ce{NaCl, CaCl2, MgSO4}\] are known as 1-1, 2-1 and 2-2 electrolytes, respectively. The value of ‘A’ is the same for all electrolytes of the same type.
संबंधित प्रश्न
State Kohlrausch’s law of independent migration of ions.
The conductivity of 0.001 mol L-1 solution of CH3COOH is 3.905× 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation (α) Given λ°(H+)= 349.6 S cm2 mol-1 and λ°(CH3COO)= 40.9S cm2mol-1.
State Kohlrausch law of independent migration of ions.
Define the following terms: Molar conductivity (⋀m)
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
| Concentration/M | 0.001 | 0.010 | 0.020 | 0.050 | 0.100 |
| 102 × κ/S m−1 | 1.237 | 11.85 | 23.15 | 55.53 | 106.74 |
Calculate `∧_"m"`for all concentrations and draw a plot between `∧_"m"`and `"c"^(1/2)`. Find the value of `∧_"m"^0`.
Write mathematical expression of molar conductivity of the given solution at infinite dilution.
The S.I. unit of cell constant for conductivity cell is __________.
Assertion: Copper sulphate can be stored in zinc vessel.
Reason: Zinc is less reactive than copper.
Which of the following increases with the increase in the concentration of the solution?
Which of the following solutions of KCl will have the highest value of molar conductivity?
