Advertisements
Advertisements
प्रश्न
Explain how, nitrogen , oxygen and argon gases are separated from air.
उत्तर
Nitrogen, oxygen and argon are separated from air by fractional distillation. Following are the steps followed to separate these gases from air:
- Air is first filtered to remove the dust particles, spores etc. Subsequently water vapours and carbon dioxide are removed.
- Air is subjected to high pressure and then cooled. This air is then allowed to expand in a chamber which causes further cooling of the air.
- This process is repeated to cool the air further. Ultimately, the air becomes so cold that it turns into a liquid.
- This liquid air is then fed into a fractionating column from its bottom end and is cooled slowly.
- Liquid nitrogen boils off first to nitrogen gas as the boiling point of nitrogen is lowest at -196°C.
- Liquid argon boils off at a boiling point of -186°C.
- Liquid oxygen boils off last as it has highest boiling point among all three (around -183°C).
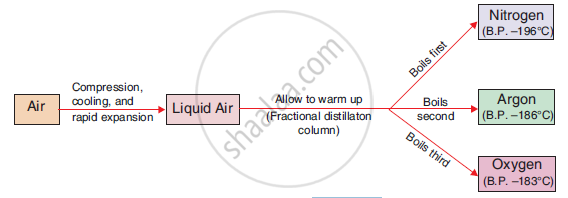
Figure 84. Separation of the major gases of air.
APPEARS IN
संबंधित प्रश्न
Define the following term of Matter .
What name is given to those elements which are neither good conductors of electricity like copper nor insulators like sulphur ?
You are given two liquids, one a solution and the other a compound. How will you distinguish the solution from the compound ?
Name a metal which can be easily cut with a knife.
Which of the following are compounds ?
- CO
- No
- NO
- Co
The element which is not common between the compounds called baking soda and soda ash is
Iron powder and sulphur powder were mixed together and divided into two parts A and B. When part A was heated strongly over a burner, then a substance C was formed. The part B was, however, not heated at all. When dilute hydrochloric acid was added to substance C, then gas D was evolved and when dilute hydrochloric acid was added to part B then gas E was evolved.
- What type of substance is B ?
- What type of substance is C ?
- Name the gas (i) D, and (ii) E ?
- State one characteristic property of gas D.
- Write one test to identify gas E.
Explain what happens when a beam of light is passed through a colloidal solution.
9.72 g of potassium chloride dissolves in 30 g of water at 70°C. Calculate the solubility of potassium chloride at that temperature.
Tincture of iodine has antiseptic properties. This solution is made by dissolving :
Describe the method of separating a mixture containing common salt, sand and ammonium chloride.
Describe the various steps involved in the separation of iodine, iron filings and salt from a mixture.
Which of the following cannot be separated from air by the process of fractional distillation ?
One of the following does not undergo sublimation. This one is :
Wipe the board with a duster and then tap the duster against the table. What do you see?
Name the metals that are used in jewellery.
State whether true or false. If false, correct the statement.
Oil and water are immiscible with each other.
Solids possess very high kinetic energy.
Complete the following table:
| S.No. | CELSIUS | KELVIN |
| 1. | 90°C | 363 K |
| 2. | ______ | 283 K |
| 3. | 63 °C | ______ |
| 4. | 250°C | ______ |
| 5. | ______ | 303 K |
The fluidity of solids: ______.
