Advertisements
Advertisements
प्रश्न
Explain the construction and working of a dry cell.
उत्तर
- A dry cell is a portable form of a leclanche cell.
- It consists of zinc vessel which acts as a negative electrode or anode.
- The vessel contains a moist paste of saw dust saturated with a solution of ammonium chloride an chloride.
- The ammonium chloride acts as an electrolyte.
- The purpose of zinc pide is to maintain the moistness of the paste being highly gyroscopic.
- The carbon rod covered with a brass cap is placed in the middle of the vessel. It acts as positive electrode or cathode.
- It is surrounded by a closely packed mixture of charcoal and manganese dioxide (Mn02) in a muslin bag.
- Here Mn02 acts as depolarizer. The zinc vessel is sealed at the top with pitch or shellac.
- A small hole is provided in it to allow the gases formed by the chemical action to escape.
- The chemical action inside the cell is the same as in avalanche cell.
APPEARS IN
संबंधित प्रश्न
Describe the construction, working and usefulness of a dry cell, with the help of a diagram.
What is solar cell?
Mention two advantages of a dry cell.
Name the scientist who invented the electric cell.
The device which converts chemical energy into electrical energy is
Secondary cells are used in ______.
______ cell is used to operate devices such as mobile phones, computers, and emergency lights.
A battery is a group of ______.
Paheli wanted to glow a torch bulb using a cell. She could not get connecting wires, instead, she got two strips of aluminium foil. Will she succeed? Explain, how?
Fig. 12.10 A and B, show a bulb connected to a cell in two different ways.
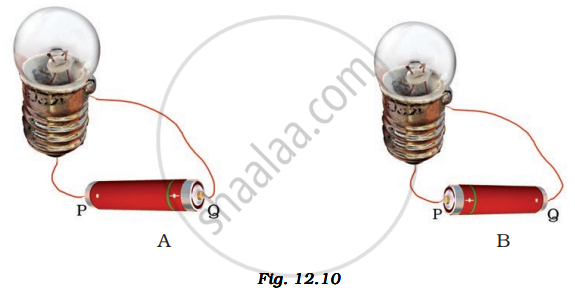
Will the bulb glow in both the cases?
