Advertisements
Advertisements
प्रश्न
Explain the significance of formal charge with the help of a suitable example
रासायनिक समीकरण/संरचनाएँ
स्पष्ट कीजिए
उत्तर
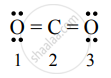
| Number of atoms | Total number of electrons in a free atom (V.E.) | Total number of non-bonding electrons (N.E.) | Total number of shared electrons in bond (B.E.) | Formal charge F.C = `("V.E.")-("N"."E")-1/2("B.E.")` |
| 1 | 6 | 4 | 4 | F.C. = `6 - 4 - 1/2 (4) = 0` |
| 2 | 4 | 0 | 8 | F.C. = `4 - 0 - 1/2 (8) = 0` |
| 3 | 6 | 4 | 4 | F.C. = `6 - 4 - 1/2 (4) = 0` |
shaalaa.com
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
