Advertisements
Advertisements
प्रश्न
Explain Tyndall effect and Brownian movement with suitable diagram.
उत्तर
1. Tyndall Effect:

When a strong beam of light is focused on a colloidal solution the path of the beam becomes visible.
This phenomenon is called as Tyndall effect. The illuminated path is called Tyndall cone. This phenomenon is due to scattering of light by colloidal particles.
2. Brownian movement:
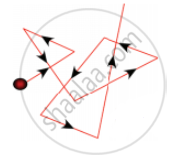
It is a kinetic property. When colloidal solution are viewed under powerful microscope, it can be seen that colloidal particles are moving constantly and rapidly in zig-zag directions. The Brownian movement of particles is due to the unbalanced bombardment of the particles by the molecules of dispersion medium.
APPEARS IN
संबंधित प्रश्न
Explain the following giving an example.
Colloid
Which of the following will show the “Tyndall effect”?
- Salt solution
- Milk
- Copper sulphate solution
- Starch solution
Name one metal and one non-metal which exist as liquids at room temperature.
Write scientific reason.
Constituent substances of a colloid cannot be separated by ordinary filtration.
Explain why particles of a colloidal solution do not settle down when left undisturbed, while in the case of a suspension they do.
The intensity of scattered light depends on the type of colloidal solution and the size of the colloidal particles.
Classify colloids based on the physical state of the dispersed phase and the dispersion medium.
A colloidal solution is a ______.
The solution of soap in water is ______.
What are Colloidal Solutions?
