Advertisements
Advertisements
प्रश्न
Find the odd one out and give its explanation.
विकल्प
Rainbow
Earthquake
Sunset
Sunrise
उत्तर
Earthquake
Explanation-
Others are due to the different phenomenon of light whereas earthquake is a natural disaster.
APPEARS IN
संबंधित प्रश्न
The specific latent heat of fusion of water is ______.
Calculate the total amount of heat energy required to convert 100 g of ice at −10℃ completely into water at 100℃. Specific heat capacity of ice = 2.1 J g-1 K-1, specific heat capacity of water = 4.2 J g-1K-1, specific latent heat of ice = 336 J g-1.
A thermally insulated pot has 150 g ice at temperature 0°C. How much steam of 100°C has to be mixed to it, so that water of temperature 50°C will be obtained? (Given : latent heat of melting of ice = 80 cal/g, latent heat of vaporization of water = 540 cal/g, specific heat of water = 1 cal/g °C)
Explain the following temperature vs time graph.
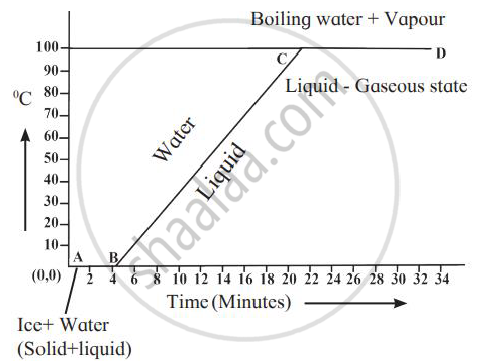
What is the name given to the energy absorbed during a phase change?
Define the term ‘specific latent heat of fusion’ of a substance.
Explain, why no tracks are left on the ice during ice skating?
Why does evaporation causes cooling and why is water used in hot water bottles?
What do you understand by the ‘latent heat of vaporization’ of a substance?
Explain the meaning of greenhouse effect.
State the main precautions to be taken in finding the latent heat of steam.
If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.
(i) Find:
(a) The heat lost by steam in terms of m1
(b) The heat gained by ice in terms of m2
(ii) Form a heat equation find the ratio of m2 : m1
Specific latent heat of vaporization of steam = 2268 kJ/kg
Specific latent heat of fusion of ice = 336 kJ/kg
Specific heat capacity of water = 4200 J/kg°C
How fog is formed?
Specific latent heat L = ______.
Who introduced the term latent heat?
600 g of copper at 50°C is mixed with lOOOg water at 20°C. Find the final temperature of the mixture. The specific heat capacity of copper is 0.4 Jg-1°C-1 and that of water is 4.2 Jg-1°C-1
Specific latent heat of a substance ______.
20 g of ice at 0°C absorbs 10,920 J of heat energy to melt and change to water at 50°C. Calculate the specific latent heat of fusion of ice. Specific heat capacity of water is 4200 J kg-1 K-1.
