Advertisements
Advertisements
प्रश्न
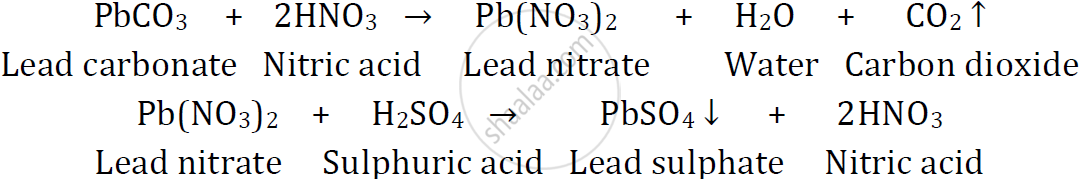
Give balanced chemical equations to prepare the following salts
Lead sulphate from lead carbonate
उत्तर
Lead sulphate from lead carbonate
APPEARS IN
संबंधित प्रश्न
The salt solution which does not react with ammonium hydroxide is :
Define normal salt.
Name a complex salt.
Identify the odd one out and justify.
Choose the correct answer from the options given below:
Which one is the acidic salt?
Salts A, B, C, D and E undergo reactions (i) to (v) respectively. Identify the anion present in these salts on the basis of these reactions. Tabulate your answers in the format given below:
(i) When silver nitrate solution is added to asolution of A, a white precipitate, insoluble in dilute nitric acid, is formed.
(ii) Addition of dilute hydrochloric acid to B produces a gas which turns lead acetate paper black.
(iii) When a freshly prepared solution of ferrous sulphate is added to a solution of C and concentrated sulphuric acid is gently poured from the side of the test, a brown ring is formed.
(iv) When dilute sulphuric acid is added to D, a gas is produced which turns acidified potassium dichromate solution from orange to green.
(v) Addition of dilute hydrochloric acid to E produces an effervescence. The gas produced turns limewater milky but does not affect acidified potassium dichromate solution.
| Salt | Anion |
| A | |
| B | |
| C | |
| D | |
| E |
State the inference drawn from the following observation :
On carrying out the flame test with a salt P a brick red flame was obtained. What is the cation in P?
Define the following and give two examples in case a normal salt .
What are double salts? Give an example?
State the terms/process for the following:
A type of salt formed by partial replacement of hydroxyl radicals with an acid radical.
