Advertisements
Advertisements
प्रश्न
Give reasons Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline
उत्तर
Nitration is usually carried out with a mixture of concentrated HNO3 and concentrated H2SO4. In the presence of these acids, most of aniline gets protonated to form anilinium ion. Therefore, in presence of acids, the reaction mixture consists of aniline and anilinium ion. Nitration of aniline due to steric hindrance at ortho position, mainly gives para nitroaniline and the nitration of anilinium ion gives m-nitroaniline. In actual practice, approximately 1:1 mixture of p-nitroaniline: m-nitroaniline is obtained.
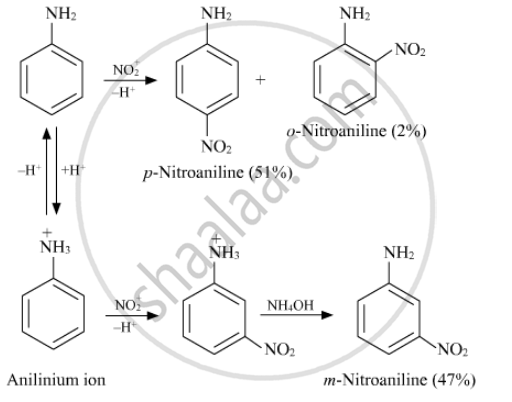
Thus, nitration of aniline gives a substantial amount of m-nitroaniline due to protonation of the amino group.
APPEARS IN
संबंधित प्रश्न
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)2CHNH2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3NHCH(CH3)2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)3CNH2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
m−BrC6H4NH2
How will you convert Ethanamine into methanamine
How will you convert Methanamine into ethanamine
Accomplish the following conversions - Aniline to 2,4,6-tribromofluorobenzene
Accomplish the following conversions - Chlorobenzene to p-chloroaniline
Complete the following reactions:
`C_6H_5NH_2 +H_2SO_4(conc.) ->`
Do the following conversions in not more than two steps :
Ethyl benzene to Benzoic acid
