Advertisements
Advertisements
प्रश्न
Identify the metallic oxide which is amphoteric in nature.
विकल्प
Calcium oxide
Barium oxide
Zinc oxide
Copper oxide
उत्तर
Zinc oxide
APPEARS IN
संबंधित प्रश्न
Answer the following in respect of element `31/15 P `
What is its valency?
The electronic configuration of an element T is 2, 8, 8, 1.
Is it a metal or a non-metal?
Arrange the elements of group 17 and group 1 according to the given conditions.
Increasing non – metallic character
Complete the following sentences
Moving across a ………….. of the periodic table the elements show increasing ………………..character (group, period, metallic, non-metallic).
A metal M forms as oxide having the formula M2O3. It belongs to third period. Write the atomic number and valency of the metal.
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 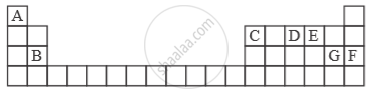
Which non-metallic element has the valency of 2?
Select the correct answer
Configuration of an element is 2, 8, 1. Which of the following statement is true?
Use the information given in (a) to (h) to identify the substances P to W selecting your answers from the given list.
List:
| Calcium | Oxygen | Copper (II) Oxide |
| Carbon | Calcium hydroxide | Copper (II) Nitrate |
| Lead (II) Oxide | Hydrogen chloride | Chlorine |
| Lead (II) Nitrate | Calcium Oxide | Ammonium chloride |
- P is white solid. When heated produces white fumes (sublime).
- P and R on warming produce an alkaline gas.
- On adding water to T, heat is evolved and R is formed.
- Q burns brightly in the air to form T.
- When S is heated, it gives off brown fumes and leaves a black residue of U.
- A solution of S is formed by warming U with dilute nitric acid.
- V is a gaseous non-metallic element that reacts with hydrogen to form W.
- A solution of W will neutralize the solution of R.
Which of the following metal (s) do not react with water?
The given table shows elements with the same number of electrons in its valence shell.
| Elements | A | B | C |
| m.p. | 63.0 | 180.0 | 97.0 |
Arrange them in order of increasing metallic character.
