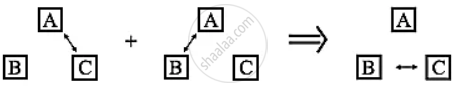
- The figure shows a schematic representation of the zeroth law of thermodynamics.
- The double arrow represents thermal equilibrium between systems.
- If systems A and C are in thermal equilibrium, and systems A and B are in thermal equilibrium, then systems B and C must be in thermal equilibrium.
- Then it can be inferred that systems A, B and C are at the same temperature.
Advertisements
Advertisements
प्रश्न
“If two systems are each in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.” This statement refers to ______.
विकल्प
zeroth law of thermodynamics
first law of thermodynamics
second law of thermodynamics
Carnot’s law
MCQ
रिक्त स्थान भरें
उत्तर
“If two systems are each in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.” This statement refers to the zeroth law of thermodynamics.
Explanation:
shaalaa.com
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
