Advertisements
Advertisements
प्रश्न
Name the following:
A metallic oxide which can be reduced into metal by hydrogen.
उत्तर
Copper oxide (CuO)
APPEARS IN
संबंधित प्रश्न
Indicate which of the following statement is true and which is false:
Zinc can liberate hydrogen from water, acid and alkali solution.
Draw a neat and well-labelled diagram for the laboratory preparation of hydrogen.
FILL IN THE BLANK
........................ zinc is preferred over pure zinc in the laboratory preparation of hydrogen.
Give reason:
Apparatus for laboratory preparation of hydrogen should be airtight and away from a naked flame.
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the addition of traces of copper [II] sulphate to the reaction medium.
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the collecting the hydrogen by downward displacement of water and not air & collecting it after all the air in the apparatus is allowed to escape.
Give balanced equation for the following conversion:
Acidified water to hydrogen – by electrolysis.
Complete and balance the equation:
[Laboratory method]
By action of dilute acid on zinc
Zinc - Zn + 2HCl → _____ + _____ [g]
Give a balanced equation for the following conversions sodium plumbite from lead.
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.
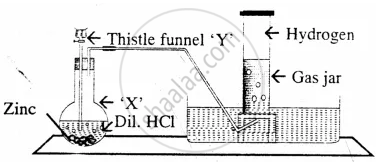
State what difference will be seen if pure zinc is added in the distillation flask ‘X’ instead of granulated zinc.
