Advertisements
Advertisements
प्रश्न
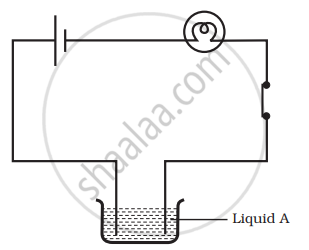
Paheli set up an experiment using liquid A in the beaker as shown in the figure. She observed that the bulb glows. Then, she replaced the liquid A by another liquid B. This time the bulb did not glow. Boojho suggested replacing the bulb by an LED. They observed that the LED glows. Explain.
उत्तर
Liquid A is a good conductor of electricity and it allows the maximum current to pass through it which is sufficient to glow the bulb.
But when it is replaced by another liquid B, bulb does not glow because the current through liquid B could be weak and therefore unable to make the bulb glow.
But the small current which is passing through B is sufficient to glow a low voltage LED, so it glows.
APPEARS IN
संबंधित प्रश्न
If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the ______ terminal of the battery.
In an electrolyte the current is due to the flow of ______.
Electroplating is an example for ______.
One of the most common applications of chemical effect of electric current is ______.
Electroplating of ______ is done on objects like water taps and cycle bell to give them a shiny appearance.
Paheli wants to deposit silver on an iron spoon. She took silver nitrate (AgNO3) solution in a beaker and set up a simple circuit for electroplating. Which terminal of the battery should the spoon be connected to? What material should the other electrode be made of?
Why is tin electroplated on iron to make cans used for storing food?
Match the following
| 1. | Anode | a. | Conducting solution |
| 2. | Cathode | b. | Positive terminal |
| 3. | Ions | c. | Negative terminal |
| 4. | Electrolyte | d. | Positively or negatively charged |
State some advantages of electroplating.
What are anodes and cathodes?
