Advertisements
Advertisements
प्रश्न
Select the ion, that would get selectively discharge from the aqueous mixture of the ions listed below :
Pb2+, Ag+, Cu+
उत्तर
Ag+
APPEARS IN
संबंधित प्रश्न
Complete the following by selecting the correct option from the choices given:
The divalent metal whose oxide is reduced to metal by electrolysis of its fused salt is __________. (Al/Na/Mg/K)
Electrolysis of aqueous sodium chloride solution will form ________ at the cathode. (Hydrogen gas / Sodium metal)
Name a non-metallic element which is a conductor of electricity.
A solution of caustic soda (NaOH) in water or when fused, conducts an electric current. what is the similarity in these two cases?
Give reasons as to why - the electrolysis of acidulated water is considered to be an example of catalysis.
Copper sulphate solution is electrolyzed using copper electrodes. Study the diagram given alongside and answer the questions that follow.
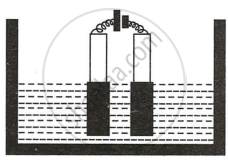
- Which electrode to your left or right is known as the oxidizing electrode and why?
- Write the equation representing the reaction that occurs.
- State two appropriate observations for the above electrolysis reactions.
Give appropriate scientific reasons for the following statement :
The electrical conductivity of acetic acid is less in comparision to the electrical conductivity of dilute sulphuric acid at a given concentration.
Give reasons why in the electroplating of an article with silver, the electrolyte sodium argentocynide solution is preferred over silver nitrate solution.
State the observation at the anode and at the cathode during the electrolysis of :
Fused lead bromide using graphite electrodes
Copper sulphate solution is electrolysed using copper electrodes.
Write the equation for the reaction occurring at the Anode electrode.
