Advertisements
Advertisements
प्रश्न
Consider the following standard electrode potential values:
\[\ce{Fe^{3+}_{ (aq)} + e^- -> Fe^{2+}_{ (aq)}}\], E0 = +0.77 V
\[\ce{MnO^{-4}_{ (aq)} + 8H^+ + 5e^- -> Mn^{2+}_{ (aq)} + 4H2O_{(l)}}\], E0 = +1.51 V
What is the cell potential for the redox reaction?
विकल्प
−2.28 V
−0.74 V
+0.74 V
+2.28 V
उत्तर
+0.74 V
Explanation:
Cell potential (E) = \[\ce{E_{cathode} − E_{anode}}\]
= 1.51 − 0.77
= 0.74 V
APPEARS IN
संबंधित प्रश्न
What are interstitial compounds?
Predict which of the following will be coloured in the aqueous solution?
Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+ and Co2+. Give reasons for each.
Calculate the number of unpaired electrons in the following gaseous ions:
Mn3+, Cr3+, V3+ and Ti3+. Which one of these is the most stable in an aqueous solution?
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number
Why does the density of transition elements increase from Titanium to Copper? (at. no. Ti = 22,
Cu = 29)
Although fluorine is more electronegative than oxygen, but the ability of oxygen to stabilise higher oxidation states exceeds that of fluorine. Why?
Assertion: \[\ce{Cu^2+}\] iodide is not known.
Reason: \[\ce{Cu^2+}\] oxidises \[\ce{I^-}\] to iodine.
Agcl is soluble in NH4OH. The solubility is due to the information of:-
Give reason for the following statement:
[Ti(H2O)]3+ is coloured while [Sc(H2O)6]3+ is colourless.
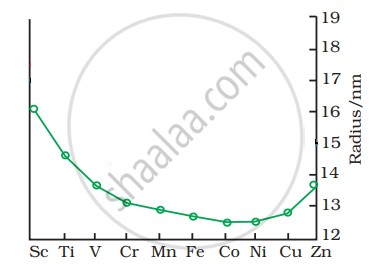
The trend of which property is represented by the following graph?