Advertisements
Advertisements
प्रश्न
Suppose a person wants to increase the efficiency of the reversible heat engine that is operating between 100°C and 300°C. He had two ways to increase efficiency.
- By decreasing the cold reservoir temperature from 100°C to 50°C and keeping the hot reservoir temperature constant
- by increasing the temperature of the hot reservoir from 300°C to 350°C by keeping the cold reservoir temperature constant.
Which is the suitable method?
उत्तर
Heat engine operates at initial temperature = 100°C + 273 = 373 K
Final temperature = 300°C + 273 = 573 K
At melting point = 273 K
Efficiency η = `1 - "T"_2/"T"_1`
= `1 - 273/573`
= 0.3491
η = 34.9%
(a) By decreasing the cold reservoir, efficiency
T1 = 300°C + 273 = 573 K
T2 = 50°C + 273 = 323 K
Efficiency η = `1 - "T"_2/"T"_1`
= `1 - 323/573`
= 0.436
η = 43.6%
(b) By increasing the temperature of hot reservoir, efficiency
T1 = 350°C + 273 = 623 K
T2 = 100°C + 273 = 373 K
Efficiency η = `1 - "T"_2/"T"_1`
= `1 - 373/623`
= 0.401
η = 40.1%
Method (a) More efficiency than method (b).
APPEARS IN
संबंधित प्रश्न
Answer in brief.
What sets the limits on the efficiency of a heat engine?
Draw a p-V diagram and explain the concept of positive and negative work. Give one example each.
The figure shows the V-T diagram for one cycle of a hypothetical heat engine which uses the ideal gas. Draw the p-V diagram diagram of the system.
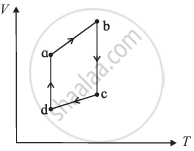
State Kelvin-Planck's statement of the second law of thermodynamics.
Define heat engine.
State the second law of thermodynamics in terms of entropy.
Derive the expression for Carnot engine efficiency.
For a heat engine operating between temperatures t1 °C and t2 °C, its efficiency will be ______.
What does a heat engine consist of?
What is a heat engine?
