Advertisements
Advertisements
प्रश्न
The decomposition of NH3 on a platinum surface is a zero-order reaction. If the rate constant (k) is 4 x 10-3 ms-1, how long will it take to reduce the initial concentration of NH3 from 0.1 M to 0.064 M?
उत्तर
Given that:
k = 4 x 10-3 Ms-1
`["A"_°]` = 0.1 M
[A] = 0.064 M
For a zero-order reaction,
k=`1/t {["A"_°]-["A"]}`
4 x 10-3 Ms-1 = `1/"t" {[0.1]-[0.064]}`
t =`( 0.1-0.064)/(4xx10^-3)=0.009xx10^3=9` seconds
संबंधित प्रश्न
The decomposition of NH3 on platinum surface is zero order reaction. What are the rates of production of N2 and H2 if k = 2.5 × 10−4 mol−1 L s−1?
The reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table:
| Experiment | A/mol L−1 | B/mol L−1 | Initial rate/mol L−1 min−1 |
| I | 0.1 | 0.1 | 2.0 × 10−2 |
| II | ______ | 0.2 | 4.0 × 10−2 |
| III | 0.4 | 0.4 | ______ |
| IV | ______ | 0.2 | 2.0 × 10−2 |
Give one example of zero order reaction.
Which of the following graphs is correct for a zero order reaction?
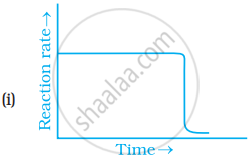
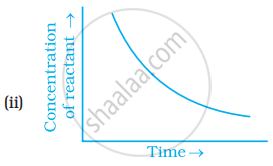
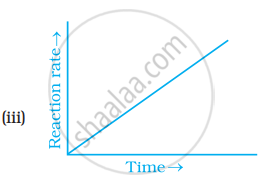

A solution with initial concentration of a mol dm-3 follow zero order kinetic. The time taken for the completion of reaction is
For a zero-order reaction, the plot of [A]t vs t is linear with a ______
If the initial concentration of substance A is 1.5 M and after 120 seconds the concentration of substance A is 0.75 M, the rate constant for the reaction if it follows zero-order kinetics is ______.
What is zeroth order reaction? Derive its integrated rate Law. What are the units of rate constant?
Derive the expression for integrated rate law for zero order reaction A → Products.
What is zero order reaction?
