Advertisements
Advertisements
प्रश्न
The net work done in the following cycle for one mol of an ideal gas will be ______ (in calorie), where in process BC, PT = constant. (R = 2 cal/mol-K).
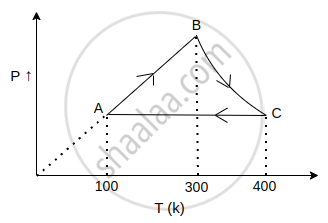
रिक्त स्थान भरें
उत्तर
The net work done in the following cycle for one mol of an ideal gas will be 200 (in calorie), where in process BC, PT = constant.
Explanation:
`"w"_"total" = "w"_"AB" + "w"_"BC" + "w"_"CA"`
`"w"_"AB" = 0` (isochoric)
`"w"_"BC" = ("nR"Delta"T")/(x - 1)` ...(Polytropic PV1/2 = constant)
`= (1 xx 2 xx 100)/(-1//2)`
= - 400 cal
`"w"_"CA" = - "nR"Delta"T"` (isobaric)
= - 1 × 2 × - 300
= 600 cal
`"w"_"total" = 0 - 400 + 600`
= 200 cal
shaalaa.com
Thermodynamics Applications - Work
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
