Advertisements
Advertisements
प्रश्न
There are three liquids A, B and C, all having different densities and different boiling points. Liquids A and C are organic in nature whereas liquid B is considered to be inorganic. When liquids A and B are put together in a container, they form a single layer. On the other hand, when, liquids B and C are mixed, they form two separate layers :
(a) Which process will you use to separate a mixture of A and B ?
(b) Which method will you use to separate a mixture of B and C ?
(c) Name the liquids which would behave like (i) A (ii) B and (iii) C.
उत्तर
- Liquid A is organic and liquid B is inorganic. When mixed, they form a single layer, i.e., they are miscible liquids. So, we will use fractional distillation to separate the mixture of liquid A and liquid B.
- Liquid B is inorganic and liquid C is organic. We will use a separating funnel to separate the mixture of liquid B and liquid C as they form two different layers.
- Liquid A is organic. When liquid A and liquid B are put together in a container, they form a single layer. So, alcohol would behave like liquid A.
- Liquid B is inorganic. So, water would behave like liquid B.
- Liquid C is organic. When liquid B and liquid C are mixed together, they form two separate layers. Oil would behave like liquid C.
APPEARS IN
संबंधित प्रश्न
Name the processes involved in two cases.
What is the scientific name of particles which make up mater?
Explain why steel is used to make railway lines.
Name two solid, two liquid and two gaseous elements at the room temperature.
Name a metal which forms amalgams.
A, B and C are all liquids. Liquid A has a comparatively low boiling point. On heating, liquid A vaporises completely without leaving behind any residue. Liquid A is being used increasingly as a fuel in motor vehicles either alone or by mixing with petrol. Liquid B has a very high boiling point. It also vaporises completely on heating, without leaving any residue. Liquid B is a conductor of electricity and used in making thermometers. Liquid C has a moderate boiling point. On heating, liquid C vaporises leaving behind a white solid D which is used in cooking vegetables. The condensation of vapours from C give a liquid E which turns anhydrous CuSO4 to blue.
(a) Which liquid could be an element ? Name this element.
(b) Which liquid could be a mixture ? Name this mixture.
(c) Which liquid could be a compound ? Name this compound.
(d) What could the solid D be ?
(e) What do you think is liquid E ?
9.72 g of potassium chloride dissolves in 30 g of water at 70°C. Calculate the solubility of potassium chloride at that temperature.
Fill in the following blanks with suitable words :
If a mixture contains iron filings as one of the constituents, it can be separated by using a .......................
Describe a method to separate a mixture of camphor and sand.
With the help of a labelled diagram, describe the method of separating ammonium chloride from a mixture of ammonium chloride and common salt. Mention the difference in the properties of ammonium chloride and sodium chloride which has made this separation possible.
The dyes present in fountain pen ink can be separated by the technique of :
The technique which is used to separate particles of a solid suspended in a liquid quickly is called :
Which one of the following scrap metal cannot be separated by magnetic separation ?
A solid mixture contains four constituents P, Q, R and S. P consists of tiny grains and it is mixed with
cement for plastering the walls. Q is a white solid which is recovered on a large scale from sea water by the
process of evaporation. R is in the form of tiny particles of a material whose corrosion is called rusting. And
S is a white solid which is used in making ordinary dry cells.
- What could P, Q, R and S be ?
- How would you separate a mixture containing P, Q, R and S ?
What difference in the properties of common salt and sand would enable you to separate a mixture of these two substances ?
Matter is made up of ______.
Justify your answer.
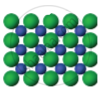
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
The arrangement of particles in three different phases of matter is shown above.
- Which state is represented by Fig. 1?
- In which state will the inter-particle attraction be maximum?
- Which one of them cannot be contained in an open vessel?
- Which one can take the shape of its container?
You want to wear your favourite shirt to a party, but the problem is that it is still wet after a wash. What steps would you take to dry it faster?
Rusting of an article made up of iron is called
______ is so hard that it can scratch glass.
