Advertisements
Advertisements
प्रश्न
Two crystals C1 and C2, made of pure silicon, are doped with arsenic and aluminium respectively.
Identify the extrinsic semiconductors so formed.
उत्तर
Arsenic is pentavalent. So, C1 doped with arsenic will form an n-type semiconductor.
Aluminium is trivalent. So, C2 doped with aluminium will form a p-type semiconductor.
APPEARS IN
संबंधित प्रश्न
Distinguish between 'intrinsic' and 'extrinsic' semiconductors
In a p-type semiconductos, which of the following statement is true:
A donor impurity results in ______.
In n-type semiconductor, the fifth electron ______.
In p-type semiconductor, the dopant is ______.
In p-type semiconductor, ______.
State how a p-type semiconductor will be obtained from a pure crystal of a semiconductor.
Sn, C, and Si, Ge are all group XIV elements. Yet, Sn is a conductor, C is an insulator while Si and Ge are semiconductors. Why?
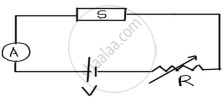
The figure shows a piece of pure semiconductor S in series with a variable resistor R and a source of constant voltage V. Should the value of R be increased or decreased to keep the reading of the ammeter constant, when semiconductor S is heated? Justify your answer
Name the extrinsic semiconductors formed when pure germanium is doped with a Pentavalent impurity. Draw the energy band diagram of extrinsic semiconductors so formed.
