Advertisements
Advertisements
प्रश्न
Two vessels separately contain two ideal gases A and B at the same temperature. The pressure of A is twice that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of molecular weights of A and B is ______.
विकल्प
2
`3/4`
`1/2`
`2/3`
MCQ
रिक्त स्थान भरें
उत्तर
Two vessels separately contain two ideal gases A and B at the same temperature. The pressure of A is twice that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of molecular weights of A and B is `underline(3/4)`.
Explanation:
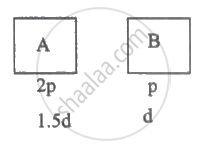
For ideal gas, PV = RT M = ρV
`therefore"PM"/rho="RT"`
`therefore("P"_"A""M"_"A")/rho_"A"=("P"_"B""M"_"B")/rho_"B"`
`"M"_"A"/"M"_"B"=rho_"A"/rhp_"B"xx"P"_"B"/"P"_"A"=3/2xx1/2=3/4`
shaalaa.com
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
