Advertisements
Advertisements
प्रश्न
Write equation for the following conversions A, B, C and D.
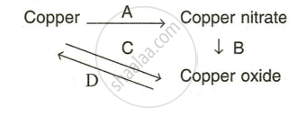
उत्तर
A: Copper can be converted into copper nitrate.
\[\ce{Cu + 4HNO3 -> \underset{Cu(NO3)2 + 2H2O + 2NO}\]
B: \[\ce{\underset{Blue crystalline}{2Cu(NO3)2} ->[\Delta] \underset{Black}{2CuO} + 4NO2 + O2}\]
C: \[\ce{\underset{Stronh heating}{2Cu + O2} ->[\Delta] \underset{Black}{2CuO}}\]
D: By reduction
\[\ce{2CuO + C ->[\Delta] 2Cu + CO2}\]
APPEARS IN
संबंधित प्रश्न
Write balanced chemical equations for Action of hot and concentrated Nitric acid on copper
What is passive iron?
Ammonia is used in the Ostwald process.
Why is quartz used in this process?
Describe the Ostwald's process for the manufacture of nitric acid with labeled diagram.
Write balanced equation and name the product formed when :
Cupric oxide reacts with nitric acid.
How will you prepare Aqua regia from nitric acid?
What happens when (Given balanced equation):
Nitric acid is added to Kl (aq)?
Complete and balance the following equation :
FeSO4 + NO + H2O → ______________
Choose the correct answer from the option given below:
The catalyst used in the manufacture of HNO3 by Ostwald process is
Fill in the blank with appropriate word/words.
98% nitric acid is obtained by distilling 68% nitric acid with ________ under ______
