Advertisements
Advertisements
प्रश्न
Write the skeleton equation for the following word equation and then balance them.
- Carbon + Oxygen → Carbon dioxide.
- Phosphorus + Chlorine → Phosphorus pentachloride.
- Sulphur + Oxygen → Sulphur dioxide.
- Magnesium + hydrogen → Magnesium + Hydrogen chloride chloride.
उत्तर
- Balanced equation:
- C + O2 → CO2
- P4 + 10 Cl2 → 4PCl5
- S + O2 → SO2
- Mg + 2HCl → MgCl2 + H2
- Skeleton equation:
- C + O2 → CO2
- P + Cl2 → PCl5
- S + O2 → SO2
- Mg + 2HCl → MgCl2 + H2
APPEARS IN
संबंधित प्रश्न
Write the balanced chemical equation for the following reaction.
\[\ce{Aluminium + Copper chloride -> Aluminium chloride + Copper}\]
One of the following is an exothermic reaction. This is:
(a) electrolysis of water
(b) conversion of limestone into quicklime
(c) process of respiration
(d) process of photosynthesis
When water is added gradually to a white solid X, a hissing sound is heard and a lot of heat is produced forming a product Y. A suspension of Y in water is applied to the walls of a house during white washing. A clear solution of Y is also used for testing carbon dioxide gas in the laboratory.
(a) What could be solid X? Write its chemical formula.
(b) What could be product Y? Write its chemical formula.
(c) What is the common name of the solution of Y which is used for testing carbon dioxide gas?
(d) Write chemical equation of the reaction which takes place on adding water to slid X.
(e) Which characteristic of chemical reactions is illustrated by this example?
Balance the following chemical equation:
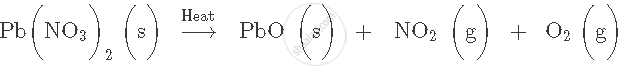
Write word equation for the following skeletal equation:
\[\ce{KClO3 -> KCl + O2}\]
Balance the following equation:
PbO + NH3 → Pb + H2O + N2
Write the balanced chemical equation of the following reaction.
sulphur + nitric acid→ sulphuric acid + nitrogen dioxide + water.
Give a balanced equation for addition of iron to copper [II] sulphate solution. State the change in colour seen.
Balance the following simple equation:
NaHCO3 + H2SO4 → Na2SO4 + H2O + CO2
The following reaction is an example of a `4"NH"_3("g") + "SO"_2 -> 4"NO"("g") + 6"H"_2"O"("g")`
- displacement reaction
- combination reaction
- redox reaction
- neutralisation reaction
