Advertisements
Advertisements
प्रश्न
1 g ice of 0℃ melts to form 1 g water at 0℃. State whether the latent heat is absorbed or given out by ice.
उत्तर
Ice turns into water when it absorbs latent heat.
APPEARS IN
संबंधित प्रश्न
What is the energy absorbed during the phase change called?
What do you understand by the term latent heat?
Write the approximate value of specific latent heat of ice.
Explain the following temperature vs time graph.
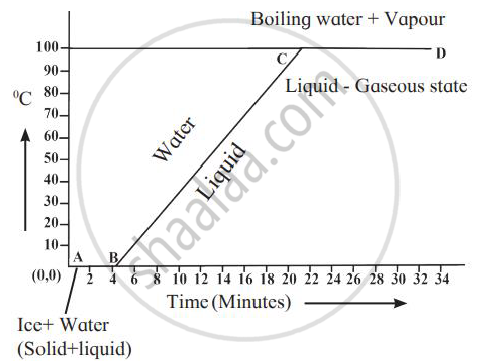
Define specific latent heat of vaporization of a substance.
Why does evaporation causes cooling and why is water used in hot water bottles?
Derive an expression for the amount of heat given out or taken up, when its temperature falls or rises by t°C.
Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C.
(Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporization of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporization = 2260 J/g, calculate:
(i) the time taken for the temperature of water to rise from 25°C to its boiling point,
(ii) the mass of water which evaporates per minute from the boiling water.
Specific latent heat of vaporisation : J/kg : : specific heat : _______
Match the columns.
| Column A | Column B |
| 1) Specific latent heat of fusion | a) Air saturated with vapour |
| 2) Specific latent heat of vaporisation | b) Solid converts into liquid |
| 3) Dew point temperature | c) liquid converts into gas |
Match the columns.
| Column A | Column B |
| 1) Absolute humidity | a) J or cal |
| 2) Latent heat | b) J/kg °C |
| 3) Specific heat capacity | c) kJ/kg |
| 4) Heat | d) no unit |
| e) kg/m3 |
During reheating, ice is converted to water at a temperature of 0 °C.
600 g of copper at 50°C is mixed with lOOOg water at 20°C. Find the final temperature of the mixture. The specific heat capacity of copper is 0.4 Jg-1°C-1 and that of water is 4.2 Jg-1°C-1
Calculate the amount of heat required to convert 200g of ice at 0°C into the water at 0°C Specific latent heat of fusion of ice = 336 Jg-1
Observe the following graph and answer the following questions:

- What does the graph represent?
- What does the line AB represent?
- What does the line BC represent?
