Advertisements
Advertisements
प्रश्न
2500 cm3 of hydrogen is taken at STP. The pressure of this gas is further increased by two and a half times (temperature remaining constant). What volume will hydrogen occupy now?
उत्तर
V1 = 2500 cm3
P1 = 1 atm = 760 mm
T1 = 273 K
V2= ?
T2 = 273 K
`"P"_2 = 760 xx 5/2 + 760 = 1900 + 760 = 2660 "mm"`
`("P"_1 "V"_1)/"T"_1 = ("P"_2 "V"_2)/"T"_2`
`(760xx2500)/273 = (2660xx"V"_2)/273`
`"V"_2 = 5000/7 = 714.29 "cm"^3`
APPEARS IN
संबंधित प्रश्न
State the law which is represented by the following graph:
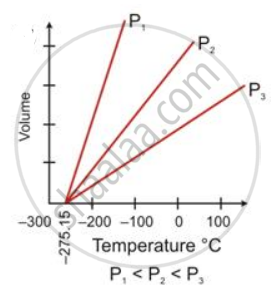
State the following:
Ice point in absolute temperature
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 temperature remaining constant?
An LPG cylinder can withstand a pressure of 14.9 atmospheres. The pressure gauge of the cylinder indicates 12 atmospheres at 27°C. Because of a sudden fire in the building, the temperature rises. At what temperature will the cylinder explode?
State Charles's law.
Give its
(i) graphical representation,
(ii) mathematical expression
(iii) significance
Fill in the blank with the correct word, from the words in option:
If the temperature of a fixed mass of a gas is kept constant and the pressure is increased, the volume correspondingly _______.
Fill in the blank with the correct word, from the words in option:
At -273°C the volume of a gas is theoretically ______.
The pressure on one mole of gas at s.t.p. is doubled and the temperature is raised to 546 K. What is the final volume of the gas ? [one mole of a gas occupies a volume of 22.4 litres at stp.]
The following question refers to one mole of chlorine gas.
What volume will it occupy at 273°C?
