Advertisements
Advertisements
प्रश्न
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction?
उत्तर
(a) Metal M is silver (Ag).
(b) Gas X2 is chlorine (Cl2).
(c) Metal salt MX is AgCl.
(d) Solution of sodium chloride and silver nitrate on mixing together can produce a precipitate of salt MX.
(e) Decomposition reaction by light takes place.
2AgCl (s) → 2Ag (s) + Cl2 (g)
APPEARS IN
संबंधित प्रश्न
What type of reaction is represented by the digestion of food in our body?
Classify the following reaction into –
- Direct combination
- Decomposition
- Displacement
- Double decomposition
The reaction is – Zinc hydroxide on heating gives zinc oxide and water.
Give a balanced equation for –
A white precipitate obtained during a double decomposition reaction involving a silver salt with sodium salt.
Electrolysis is type of ______ reaction.
Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
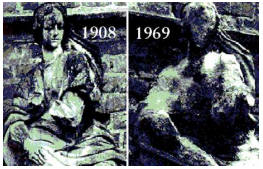
Marble statues are corroded or stained rain water. Identify the main reason.
The reaction of calcium carbonate, with freshly prepared lime water, is shown below:
Answer the questions with the help of a diagram:
- What type of reaction does calcium carbonate undergoes?
- What change in colour is observed in lime?
- Write the chemical equation.


- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
What is observed when silver chloride is exposed to sunlight? Give the type of reaction involved.
A solution of a substance ‘X’ is used for whitewashing.
- Name the substance ‘X’ and write its formula.
- Write the reaction of the substance ‘X’ named in the above formula with water.
