Advertisements
Advertisements
प्रश्न
A sample of ferrous oxide has actual formula Fe0.93O1.00. In this sample what fraction of metal ions are Fe2+ ions? What type of nonstoichiometric defect is present in this sample?
उत्तर
Let the formula of sample be (Fe2+) × (Fe3+)yO.
On looking at the given formula of the compound
x + y = 0.93 ......(1)
Total positive charge on ferrous and ferric ions should balance the two units of negative charge on oxygen. Therefore,
2x + 3y = 2 .....(2)
⇒ `x + 3/2 y` = 1 ......(3)
On subtracting equation (1) from equation (3) we have
`3/2 y - y` = 1 – 0.93
⇒ `1/2 y` = 0.07
⇒ y = 0.14
On putting the value of y in equation (1) we get,
x + 0.14 = 0.93
⇒ x = 0.93 – 0.14
x = 0.79
Fraction of Fe2+ ions present in the sample = `0.79/0.93` = 0.81
Metal deficiency defect is present in the sample because iron is less in amount than that required for stoichiometric composition.
APPEARS IN
संबंधित प्रश्न
If NaCl is doped with 10−3 mol % of SrCl2, what is the concentration of cation vacancies?
Yellow colour of NaCl crystals in sodium vapour is due to ____________.
The following diagram shows:
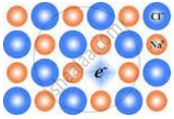
Which of the following will have metal deficiency defect?
Which of the following is a non-stoichiometric defect?
Pink colour of LiCl crystals is due to ______.
Why does table salt, NaCl, some times appear yellow in colour?
Why does white ZnO (s) becomes yellow upon heating?
Match the types of defect given in Column I with the statement given in Column II.
| Column I | Column II |
| (i) Impurity defect | (a) NaCl with anionic sites called F-centres |
| (ii) Metal excess defect | (b) FeO with Fe3+ |
| (iii) Metal deficiency defect | (c) NaCl with Sr2+ and some cationic sites vacant |
The non-stoichiometric compound Fe0 940 is formed when x% of Fe2+ ions are replaced by as many `2/3` Fe3+ ions. The value of x is
