Advertisements
Advertisements
प्रश्न
Account for the following:
Ethylamine is soluble in water whereas aniline is not.
उत्तर १
Ethylamine is soluble in water whereas aniline is not:
Ethylamine when added to water forms intermolecular H−bonds with water. Hence, it is soluble in water.
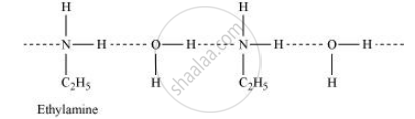
But aniline does not undergo H−bonding with water to a very large extent due to the presence of a large hydrophobic −C6H5 group. Hence, aniline is insoluble in water.
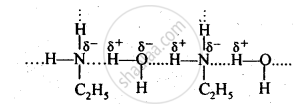
उत्तर २
Ethylamine dissolves in water due to intermolecular H-bonding. However, in case of aniline, due to the large hydrophobic part, i.e., hydrocarbon part, the extent of H-bonding is very less therefore aniline is insoluble in water.
APPEARS IN
संबंधित प्रश्न
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)2CHNH2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3(CH2)2NH2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
C6H5NHCH3
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
m−BrC6H4NH2
Give one chemical test to distinguish between the following pair of compounds.
Aniline and benzylamine
Give one chemical test to distinguish between the following pair of compounds.
Aniline and N-methylaniline
How will you convert ethanoic acid into methanamine?
Accomplish the following conversions - Aniline to benzyl alcohol.
An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B and C.
Complete the following reactions:
`C_6H_5NH_2 + Br_2(aq) ->`
Complete the following reactions:
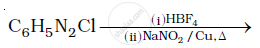
Do the following conversions in not more than two steps :
Ethyl benzene to Benzoic acid
Do the following conversions in not more than two steps :
Propanone to Propene
Write the structure of 2,4-dinitrochlorobenzene
How are ethylamine and ethyl methyl amine distinguished by using nitrous acid?
Identify the incorrect IUPAC name.
An organic compound (A) with molecular formula C3H7NO on heating with Br2 and KOH forms a compound (B). Compound (B) on heating with CHCl3 and alcoholic KOH produces a foul-smelling compound (C) and on reacting with C6H5SO2Cl forms a compound (D) which is soluble in alkali. Write the structure of (A), (B), (C) and (D).
