Advertisements
Advertisements
प्रश्न
An element Y has a valency of 4. Write the formula for its :
- chloride
- oxide
- sulphate
- carbonate
- nitrate
उत्तर
Formula for the listed compounds are as follows:
- Crossing the valencies, chloride for the element Y will have the chemical formula YCl4.

- Crossing over the valencies, oxide for the element Y will have the chemical formula YO2.
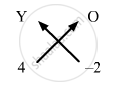
- Crossing the valencies, sulphate for the element Y will have the chemical formula Y(SO4)2.

- Crossing the valencies, carbonate for the element Y will have the chemical formula Y(CO3)2.

- Crossing the valencies, nitrate for the element Y will have the chemical formula Y(NO3)4.
APPEARS IN
संबंधित प्रश्न
State the chemical symbols for the following elements :
Sodium, Potassium, Iron, Copper, Mercury, Silver.
Name the following compounds. Also write the symbols/formulae of the ions present in them :
- CuSO4
- (NH4)2SO4
- Na2O
- Na2CO3
- CaCl2
An element A forms an oxide A2O5.
What will be the formula of chloride of A ?
Complete the following statement by selecting the correct option :
The correct formula of aluminium oxide is
Correct the following statement:
Formula of iron (lll) oxide is FeO.
Name the element in the compound and give the formula of the following compound:
Acetic acid
Write the formula of the following compound:
Calcium sulphite
Write the formula of the following compound:
Zinc sulphate
Give the chemical formulae for the following compounds and compute the ratio by mass of the combining elements in each one of them. (You may use appendix-III).
- Ammonia
- Carbon monoxide
- (Hydrogen chloride
- Aluminium fluoride
- Magnesium sulphide
Write the steps that are followed to write down the chemical formula of a substance.
