Advertisements
Advertisements
प्रश्न
Explain why high pressure is required in the manufacture of sulphur trioxide by the contact process. State the law or principle used.
टीपा लिहा
उत्तर
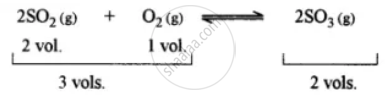
This is because the forward reaction is accompanied by a decrease in volume.
The principle used is i.e., Chatelier’s principle.
It states that when a system in equilibrium is subjected to stress, (i.e., change of concentration, temperature pressure etc.), the equilibrium tends to shift in a direction so as to undergo the effect of applied stress.
shaalaa.com
Reversible Reactions and Dynamic Equilibrium - The Contact Process for the Manufacture of Sulphuric Acid
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
