Advertisements
Advertisements
प्रश्न
Explain with a diagram the preparation of aqueous ammonia.
उत्तर
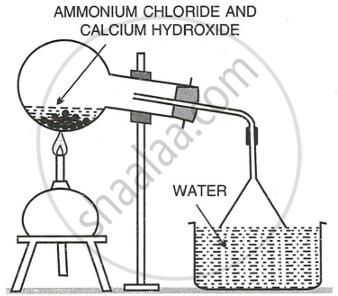
Preparation of Aqueous Ammonia: An aqueous solution of ammonia is prepared by dissolving ammonia in water. The rate of dissolution of ammonia in water is very high; therefore, back suction of water is possible. To avoid this, a funnel is attached to the outer end of the delivery tube with rubber tubing (the process is similar to the method used to change \[\ce{HCl}\] gas into hydrochloric acid).
Procedure: Water is taken in a container, and only a small portion of the mouth of the funnel is dipped in water.
As ammonia dissolves in water at a higher rate than its production in the flask, the pressure in the funnel above water level decreases for a moment, and water rushes into the funnel. As a result, the rim of the funnel loses its contact with water. Since ammonia produced pushes the water down, the funnel comes in contact with water again. In this way, ammonia dissolves in water without back suction of water.
 |
| Preparation of aqueous solution of ammonia |
APPEARS IN
संबंधित प्रश्न
Identify the cations in the following case :
NH4OH Solution, when added to the Solution (B), gives white ppt which does not dissolve in excess.
Identify the cations in the following case:
NaOH Solution, when added to Solution (C), gives white ppt which is insoluble in excess
Distinguish between the given pair of compounds using the test given within brackets:
Iron (II) sulphate and iron (III) sulphate (using ammonium hydroxide)
State your observation When calcium hydroxide is heated with ammonium chloride crystals.
What do you observe when ammonium hydroxide is added to the aqueous solution of Iron (III) chloride.
Why is ammonium hydroxide used in qualitative analysis? Give two equations to justify your answer.
When ammonium hydroxide is added to solution B, a pale blue precipitate is formed. This pale blue precipitate dissolves in excess ammonium hydroxide giving an inky blue solution. What is the cation [positive ion] present in solution B? what is the probable colour of solution B.
Q. 24
Write a balanced chemical equation for reaction of ammonia with heated copper oxide
Distinguish by a chemical test, sulphuric acid from nitric acid and hydrochloric acid.
