Advertisements
Advertisements
प्रश्न
For a zero order reaction will the molecularity be equal to zero? Explain.
उत्तर
The molecularity of any reaction is the number of reacting species taking part in an elementary reaction. The zero molecularity means there is no reactant so the reaction does not occur. Therefore molecularity cannot be zero for a reaction.
APPEARS IN
संबंधित प्रश्न
For which of the following reaction the units of rate constant and rate of the reaction are same?
Which of the following graphs is correct for a zero order reaction?
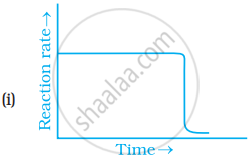
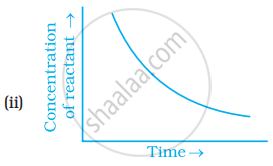
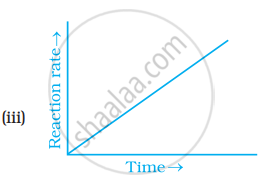

Derive an expression to calculate time required for completion of zero order reaction.
A solution with initial concentration of a mol dm-3 follow zero order kinetic. The time taken for the completion of reaction is
Consider the following statement:-
(i) Increase in concentration of reactant increases the rate of a zero-order reaction.
(ii) Rate constant k is equal to collision frequency A if Ea = 0
(iii) Rate constant k is equal to collision frequency A if Ea = 0
(iv) In k vs t is a straight line
(v) In k vs 1/T is a straight line
Which of the above statement is correct?
For a zero-order reaction, the plot of [A]t vs t is linear with a ______
Write the expression of integrated rate equation for zero order reaction.
The following experimental rate data were obtained for a reaction carried out at 25°C:
\[\ce{A_{(g)} + B_{(g)} -> C_{(g)} + A_{(g)}}\]
| Initial [A(g)]/mol dm−3 | Initial [B(g)]/mol dm−3 | Initial rate/mol dm−3s−1 |
| 3.0 × 10−2 | 2.0 × 10−2 | 1.89 × 10−4 |
| 3.0 × 10−2 | 4.0 × 10−2 | 1.89 × 10−4 |
| 6.0 × 10−2 | 4.0 × 10−2 | 7.56 × 10−4 |
What are the orders with respect to A(g) and B(g)?
What is zero order reaction?
If unit of rate constant is mol dm−3s−1, the order of reaction would be ______.
