Advertisements
Advertisements
प्रश्न
Give examples for the following:
Two solid covalent compounds.
उत्तर
Urea, Glucose.
APPEARS IN
संबंधित प्रश्न
Give one example of a molecule containing a single covalent bond.
What type of chemical bonds are formed by carbon? Why?
State one test by which sodium chloride can be distinguished from sugar.
State the type of bond formed when the combining atom has small E.N. difference.
Potassium (Atomic No. 19) and chlorine (Atomic No. 17) react to form a compound. On the basis of electronic concept, explain
(i) oxidation
(ii) reduction
(iii)oxidising agent
(iv)reducing agent
Name two compounds that are covalent when taken pure but produce ions when dissolved in water.
Fill in the blank and rewrite the completed statement:
Covalent compounds are generally soluble in _________ solvents.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
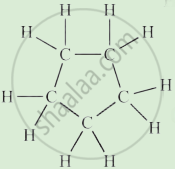 |
Which of the following statements are correct for carbon compounds?
- Most carbon compounds are good conductors of electricity.
- Most carbon compounds are poor conductors of electricity.
- Force of attraction between molecules of carbon compounds is not very strong.
- Force of attraction between molecules of carbon compounds is very strong.
Assertion (A): Melting point and boiling point of ethanol are lower than that of sodium chloride.
Reason (R): The forces of attraction between the molecules of ionic compounds are very strong.
