Advertisements
Advertisements
प्रश्न
Give a simple chemical test to distinguish between the following pair of compounds :
CH3CH2CHO and CH3CH2COCH3
उत्तर
2-Butanone has a methyl group attached to carbonyl carbon unlike propanal. Hence, it can give iodofom test.

संबंधित प्रश्न
Give a simple chemical test to distinguish between the following pair of compounds:
Ethanal and Propanal
Distinguish between:
C6H5-COCH3 and C6H5-CHO
A and B are two functional isomers of compound C3H6O.On heating with NaOH and I2, isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?
An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acid. Write the possible structure of the compound.
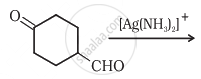
Complete the synthesis by giving missing starting material, reagent or product.
Alkenes decolourise bromine water in presence of CCl4 due to formation of ______.
The correct set of products obtained in the following reactions:
- \[\ce{RCN ->[reduction]}\]
- \[\ce{RCN ->[(i) CH3MgBr][(ii) H2O]}\]
- \[\ce{RNC ->[hydrolysis]}\]
- \[\ce{RNH2 ->[HNO2]}\]
You are given four organic compounds “A”, “B” , “C” and “D”. The compounds “A”, “B” and “C” form an orange-red precipitate with 2, 4 DNP reagent. Compounds “A” and “B” reduce Tollen’s reagent while compounds “C” and “D” do not. Both “B” and “C” give a yellow precipitate when heated with iodine in the presence of NaOH. Compound “D” gives brisk effervescence with sodium bicarbonate solution. Identify “A”, “B”, “C” and “D” given the number of carbon atoms in three of these carbon compounds is three while one has two carbon atoms. Give an explanation for our answer.
