Advertisements
Advertisements
प्रश्न
In the presence of peroxide addition of HBr to propene takes place according to anti Markovnikov’s rule but peroxide effect is not seen in the case of HCl and HI. Explain.
उत्तर
The bond energy of HCl is higher than that of HBr thus it is not cleaved by free radical mechanism to exhibit peroxide effect. However in case of HI the bond energy is so low that the iodine radical forms readily and after formation it combines to form an iodine molecule.
APPEARS IN
संबंधित प्रश्न
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
Write a chemical equation for combustion reaction of the following hydrocarbon:
Butane
Write a chemical equation for combustion reaction of the following hydrocarbon:
Pentene
Write a chemical equation for combustion reaction of the following hydrocarbon:
Hexyne
Write a chemical equation for combustion reaction of the following hydrocarbon:
Toluene
Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
Which of the following alkenes on ozonolysis give a mixture of ketones only?
| (i) | CH3 – CH = CH – CH3 |
| (ii) | \[\begin{array}{cc} \ce{CH3 - C - CH = CH2}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | 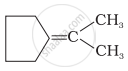 |
| (iv) | \[\begin{array}{cc} \phantom{...................}\ce{CH3}\\ \phantom{..............}/\\ \ce{(CH3)2 C = C}\\ \phantom{..............}\backslash\\ \phantom{...................}\ce{CH3} \end{array}\] |
An alkene 'X' on ozonolysis produces two moles of isovaleraldehyde. Predict the IUPAC name of the alkene.
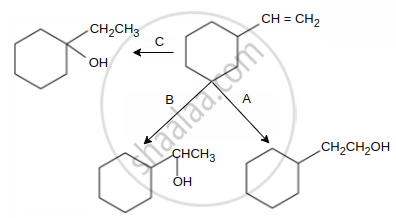
Select schemes A, B, C out of
(I) acid catalysed hydration
(II) HBO
(III) oxymercuration-demercuration
3-Methyl-pent-2-ene on reaction with HBr in presence of peroxide forms an addition product. The number of possible stereoisomers for the product is ______.
