Advertisements
Advertisements
प्रश्न
In uranium (Z = 92) the K absorption edge is 0.107 Å and the Kα line is 0.126 Å, and the wavelength of the L absorption edge is ______.
पर्याय
0.7 Å
1 Å
2 Å
3.2 Å
MCQ
रिकाम्या जागा भरा
उत्तर
In uranium (Z = 92) the K absorption edge is 0.107 Å and the Kα line is 0.126 Å, and the wavelength of the L absorption edge is 0.7 Å.
Explanation:
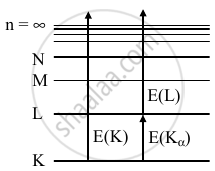
E(K) = `(hc)/(lambda_K)`
= `12.4/0.107` = 115.9 keV
E(Kα) = E(K) - E(L)
= `(hc)/(lambda_alpha)` = 98.4 keV
EL = E(K) – E(Kα)
= 115.4 - 98.4
EL = 17.5 keV
`lambda_L = (hc)/(EL)`
= `(12.4 keVÅ)/(17.5keV)`
= 0.709 Å
shaalaa.com
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
