Advertisements
Advertisements
प्रश्न
Is burning a physical change or a chemical change? Why?
उत्तर
Burning is a chemical change as new substance is formed with new properties and it cannot be reversed.
APPEARS IN
संबंधित प्रश्न
When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Fill in the blank
change in which the substance can be brought back to its original state is called a ............... change.
Write true or false for the statement.
Dissolving of solute in a solvent and rusting of iron are both physical changes.
Solve this crossword by using the clues that follow.
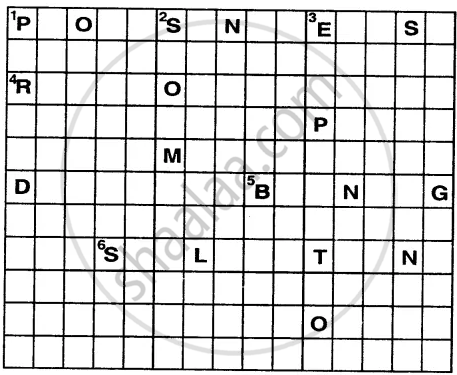
Across
1. Energy in the form of sunlight is absorbed by the green plants in this process.
4. The reddish-brown substance formed over iron in the presence of oxygen and moisture.
5. This change is permanent and irreversible.
6. The process in which a solid directly changes into gaseous state.
Down
1. It is the change that takes place in case of swinging pendulum of a clock.
2. Occurrence of this is a non-periodic change as well as a natural change.
3. It is a physical change
Fill in the blank
Revolution of the earth around the sun is a ________ change.
What is a physical change ? Give two examples of physical changes.
Is a change of state of matter – a physical or a chemical change. Give reasons.
Wood to sawdust:______:: Wood to Ash: Chemical change
Higher order Thinking question
Boiling of water is a physical change; but boiling of egg is a chemical change. Why?
Two drops of dilute sulphuric acid were added to 1 g of copper sulphate powder and then a small amount of hot water was added to dissolve it (step I). On cooling, beautiful blue coloured crystals got separated (step II). Step I and Step II are:
