Advertisements
Advertisements
प्रश्न
Justify with reason, whether the following nuclear reactions are allowed or not.
\[\ce{^A_Z X -> ^A_{Z - 2}X + ^4_{2}He}\]
उत्तर
This reaction is not allowed because even though charge (atomic number) is conserved in it, the mass number [= A on the L H.S. and equal to (A + 4)] on the R.H.S. is not conserved.
APPEARS IN
संबंधित प्रश्न
State three factors on which the rate of emission of electrons from a metal surface depends
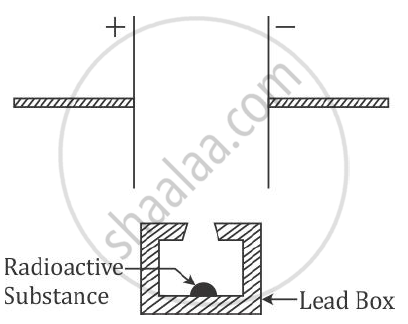
Complete the diagram as given above by drawing the deflection of radioactive radiations in an electric field
An electromagnetic radiation is used for photography in fog. [2]
(i) Identify the radiation.
(ii) Why is this radiation mentioned by you, ideal for this purpose ?
What kind of change takes place in a nucleus when a β -particle is emitted?
Explain the use of radioactive in the field of medicine, agriculture and industry.
A nucleus of an element X which has the symbol `""_84^202` X emits an alpha particle and then a beta particle. The final nucleus is `""_"b"^"a"` Y Find a and b.
What are the types of emission?
Explain, why radium paint, consisting of zinc sulphide and a trace of radium salt, glows in the dark?
The activity of a certain radionuclide decreases to 15 percent of its original value in 10 days. What is its half life?
[ln (0.15) = -1.9]
