Advertisements
Advertisements
प्रश्न
Name two colourless metal ions.
उत्तर १
Na+, Ca2+
उत्तर २
Potassium ion (K+), Sodium ion (Na+)
APPEARS IN
संबंधित प्रश्न
A compound X (having the vinegar-like smell) when treated with ethanol in the presence of the acid Z, gives a compound Y which has a fruity smell.
The reaction is:
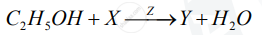
1) Identify Y and Z.
2) Write the structural formula of X.
3) Name the above reaction.
Name two coloured metal ions.
Name two bases which are not alkalis but dissolves in strong alkalis.
Write balanced equation for a metal that evolves a gas which burns with a pop sound when boiled with alkali solutions.
What do you observe when freshly precipitated aluminium hydroxide reacts with caustic soda solution? Give balanced equation.
What do you understand by amphoteric oxide? Give the balanced equations for the reaction with three different amphoteric oxides with a caustic alkali. Write your observation if any.
Name: Two coloured ions
Fill in the blank.
The hydroxide which is soluble in excess of NaOH is _________ [Zn(OH)2 / Fe(OH)3 / Fe(OH)2].
Write the equation for the following reaction :
Zinc oxide is treated with sodium hydroxide solution.
Write balanced equations for a Metal that Evolves a Gas Which Burns with a Pop Sound When Boiled with Alkali Solutions.
